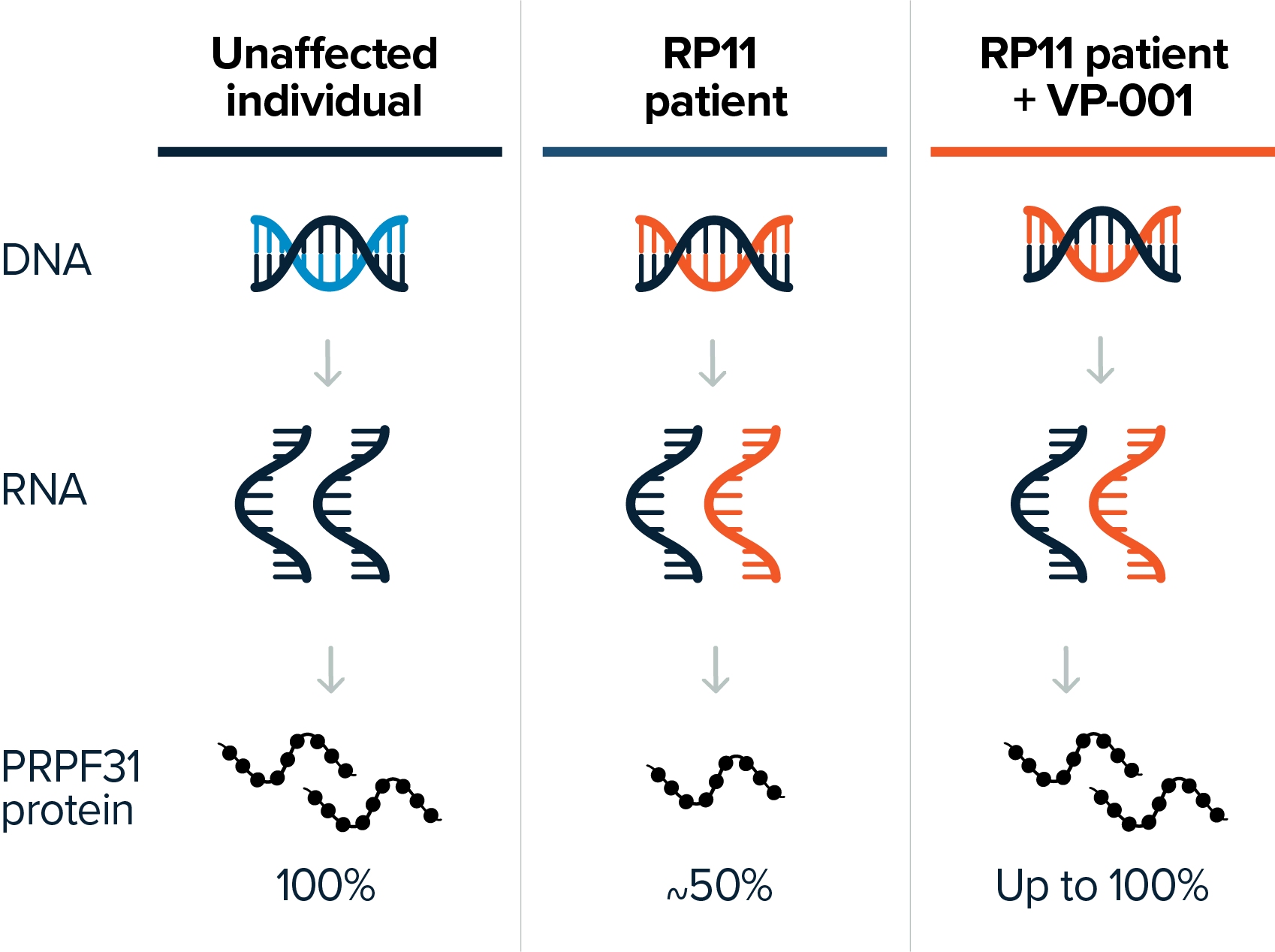
RP11 is a devastating, progressive and blinding eye disease caused by under-expression of the PRPF31 gene.
Patients experience vision changes from childhood, leading to legal blindness by 40–50 years of age. Disease management consists of visual aids and genetic counselling, but there are currently no available treatments for RP11 that treat the course of the disease.
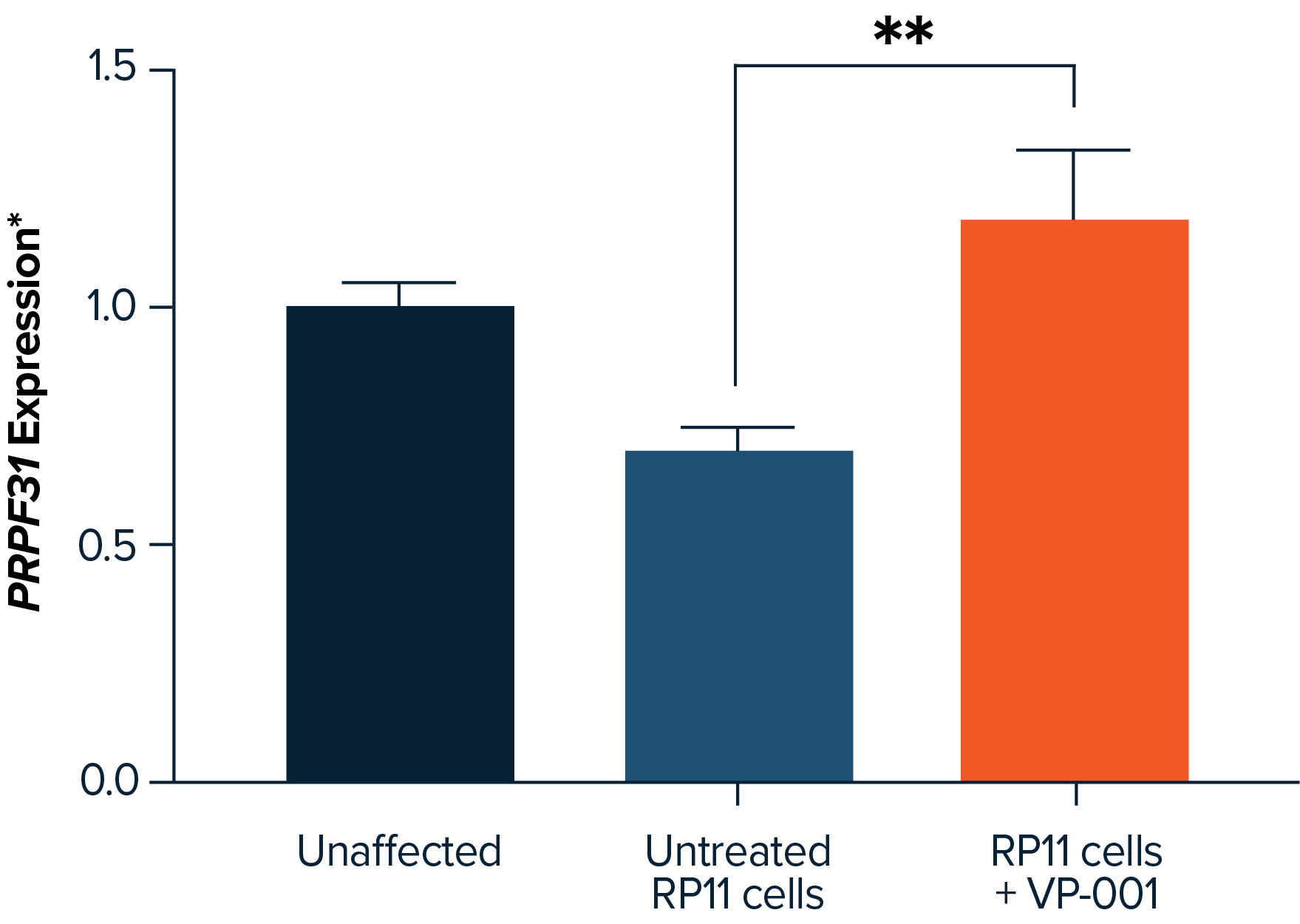
PYC has developed VP-001, a therapy that addresses the underlying genetic cause of RP11 and has achieved breakthrough pre-clinical results in the rescue of the appearance of affected retinal cells in patient-derived models. VP-001 is the first drug candidate to enter clinical trials for RP11 and is currently in a mid-stage clinical study in RP11 patients. VP-001 has provided promising safety and efficacy insights from RP11 patients dosed in this Phase 1 study.