A new generation of life-changing RNA therapeutics
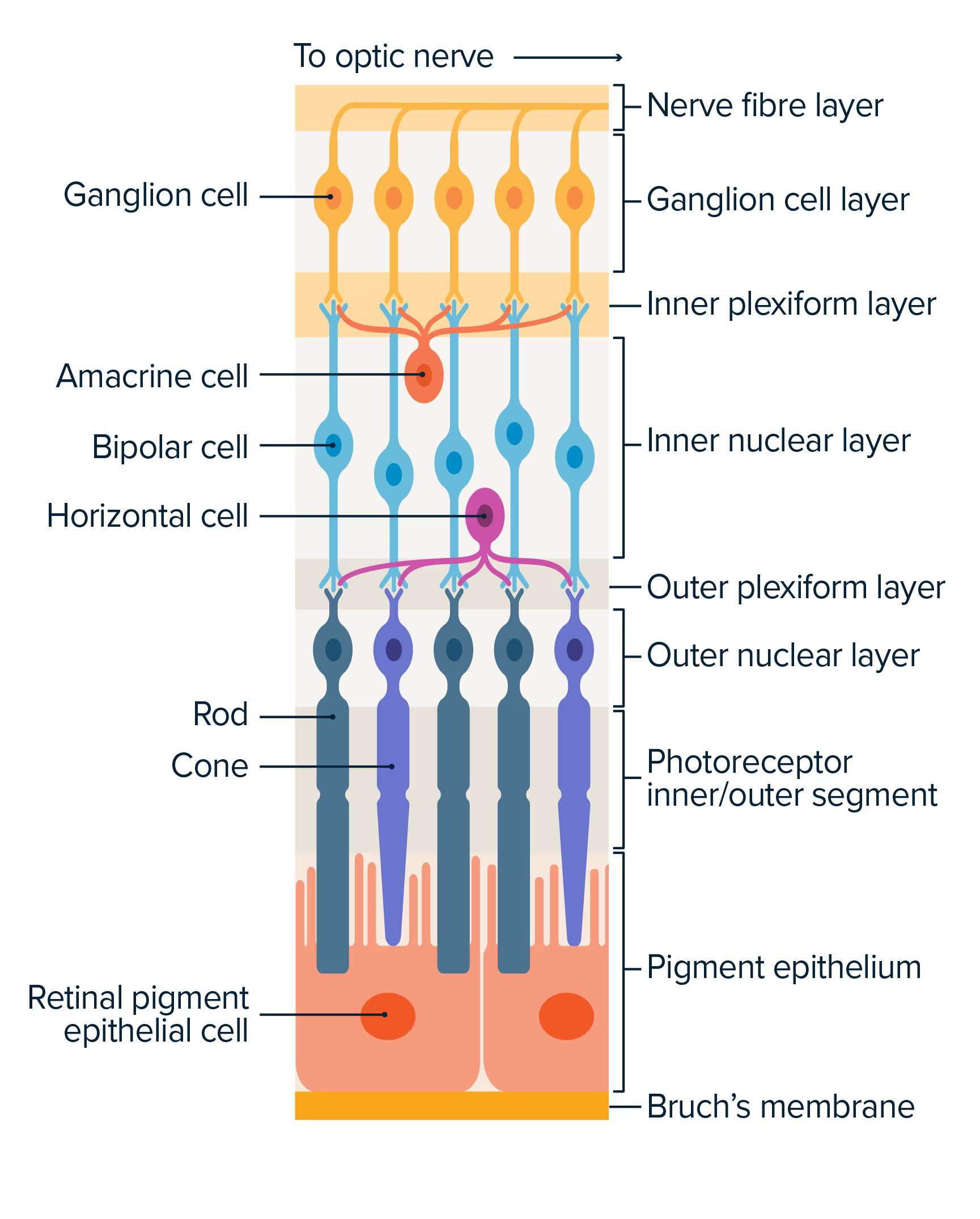
RNA therapeutics treat disease at the cellular level – they are precision therapies that have the potential to treat disease by instructing the body to modify protein production to allow for proper cell function.
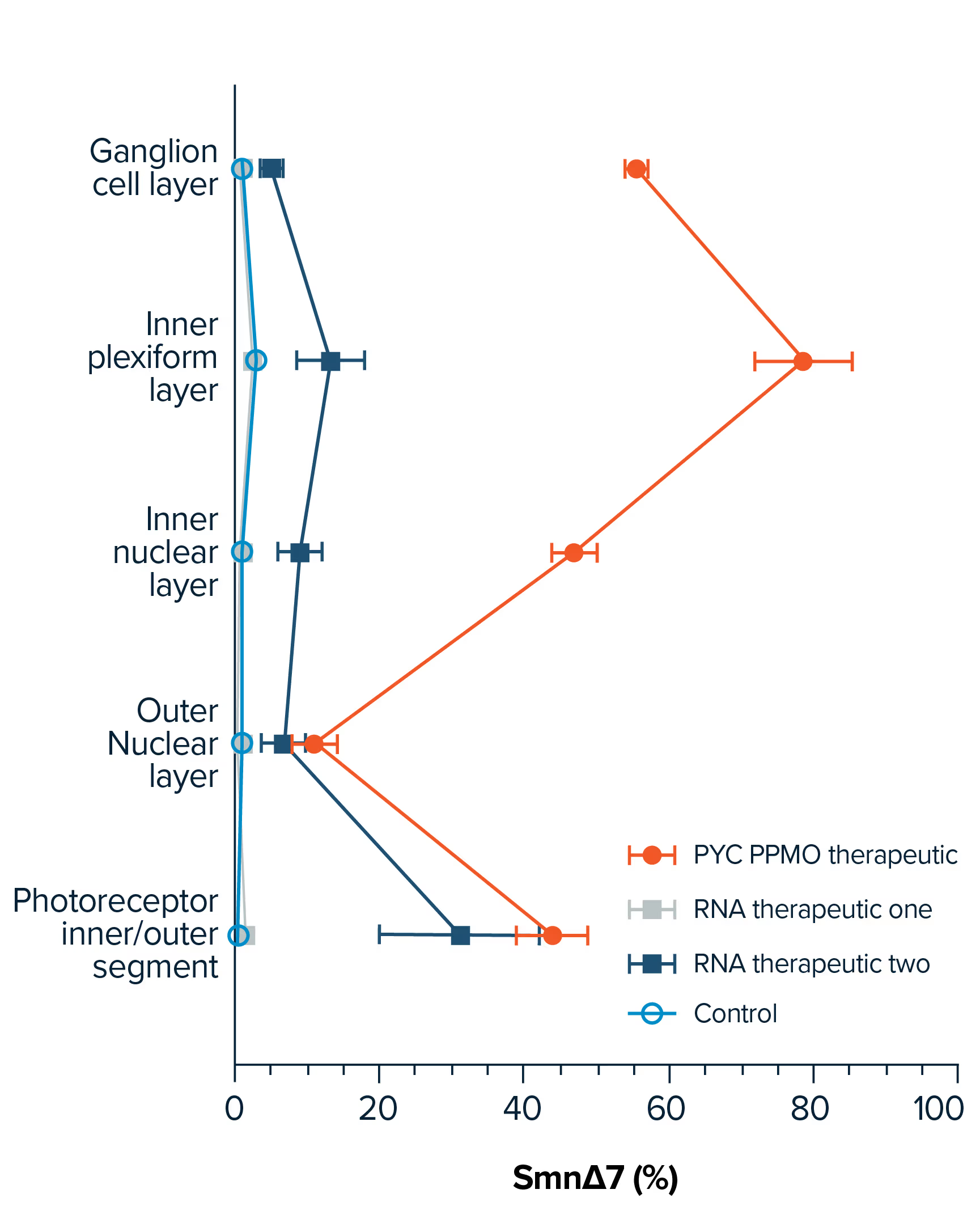
Commercialised RNA treatments have been restricted by their poor uptake into the target cell as a result of their size and molecular structure. This ‘delivery challenge’ has limited the number of diseases for which RNA therapies have been successfully created.
By binding our custom RNA therapies with our proprietary therapeutic delivery platform of cell-penetrating peptides (CPPs), we are able to solve this therapeutic challenge to provide targeted treatments to a wider range of diseases.