VP-001
Retinitis Pigmentosa Type 11 (RP11)
A severe progressive and blinding eye disease
Degenerative sight of an RP11 patient

Child is noticed to be clumsy especially at night leading to diagnosis

Patients experience night blindness and increasing tunnel vision as peripheral vision begins to decline

Patients diagnosed with legal blindness around 5th decade of life significantly impacting quality of life
Unaffected individual |
RP11 patient |
||
|---|---|---|---|
|
|
|
DNA |
RP11 patients have a mutation in one copy of the PRPF31 gene |
|
|

|
RNA |
The mutation leads to an unstable RNA message that is rapidly degraded |
|
|

|
PRPF31
|
This leads to cells in the retina having half as much PRPF31 protein as they require to function normally |
100% |
~50% |
Unaffected individual
-
DNA




-
RNA




-
Protein


Functional PRPF31 expression = 100%
2 'healthy' copies of the PRPF31 gene results in sufficient PRPF31 protein expression
RP11 patient
-
DNA




-
RNA




-
Protein


Functional PRPF31 expression = ~50%-70%
RP11 patients have a mutation in one copy of the PRPF31 gene, leading to retinal cells having half as much PRPF31 protein required for normal function
RP11 patient with single dose of VP-001
-
DNA




-
RNA




-
Protein


Restoration of PRPF31 expression towards 100%
VP-001 increases expression of the remaining healthy copy of PRPF31 to compensate for the protein insufficiency
Patient derived models provide early insight into the efficacy of VP-001 in visibly restoring the appearance of diseases cells
Recent advances in technology allow PYC to grow the affected cells of a patient that carries the targeted disease
The diseased cells are then treated with PYC’s drug candidate to evaluate the effect of the drug
The results of these studies give PYC the greatest available insight on efficacy of the drug prior to dosing patients – a significant removal of risk from the drug approval process.
VP-001 visibly improves the appearance of the cells affected in RP11 in ‘retina in a dish’ models
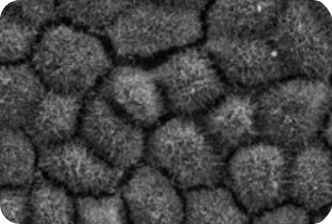
1. AN ‘UNAFFECTED’ INDIVIDUAL
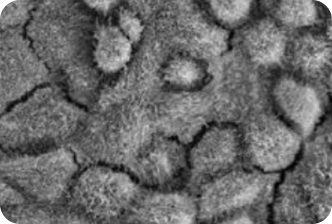
2. A PATIENT WITH RP11
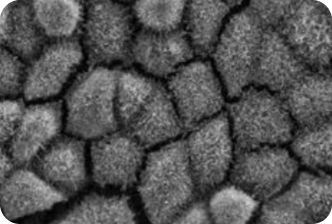
3. A PATIENT WITH RP11 AFTER
A SINGLE DOSE OF VP-001
VP-001 restores patient-derived RPE cells back to the appearance of cells from unaffected individuals

Patient Trials
PYC is currently conducting patient trials. Please contact us for more information.
Register your interest Patient Trials