We are committed to applying our breakthrough RNA technology to the development of life-changing therapies.
We are initially focused on three core therapeutic areas – the ocular, central nervous and renal systems – although our proprietary drug delivery platform has the potential to revolutionise treatment for many genetic diseases.
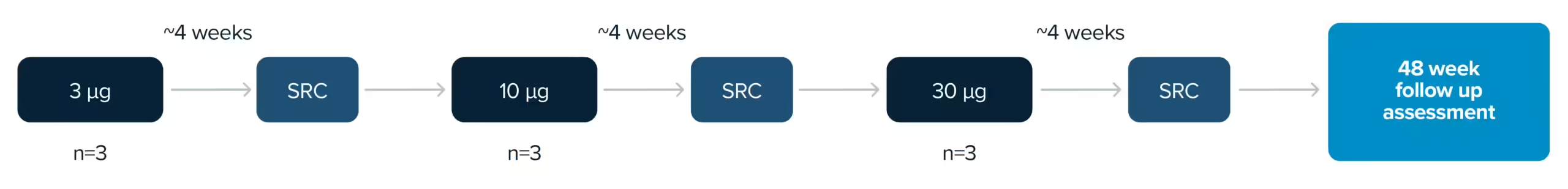
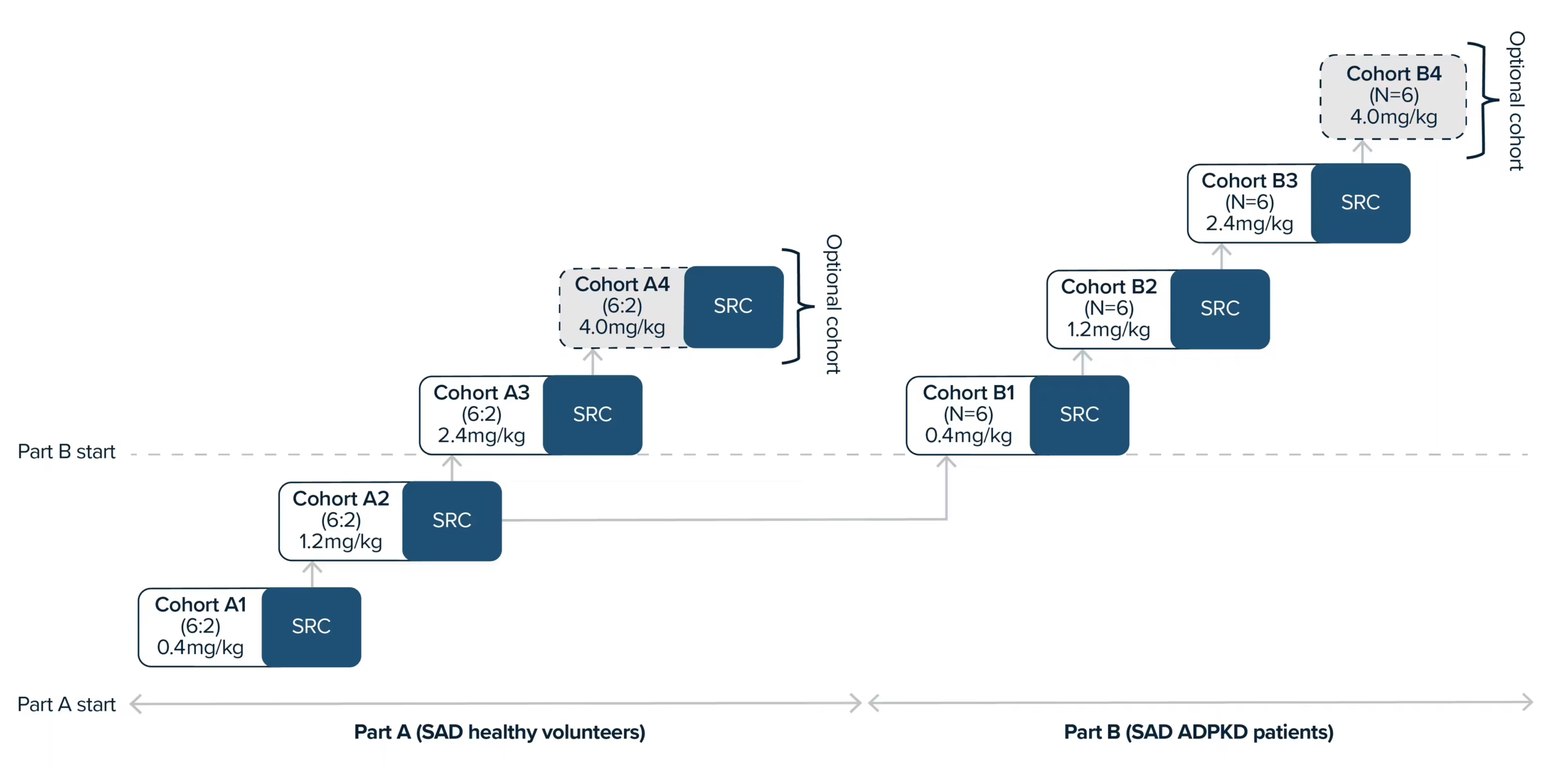
Patient trials are essential to the development of all novel therapeutics. At PYC, we conduct responsible patient trials to ensure that our treatments are safe and effective. These trials rely on patient volunteers and are crucial to the development of disease-modifying therapies for genetic diseases.